What is the Essure Lawsuit? 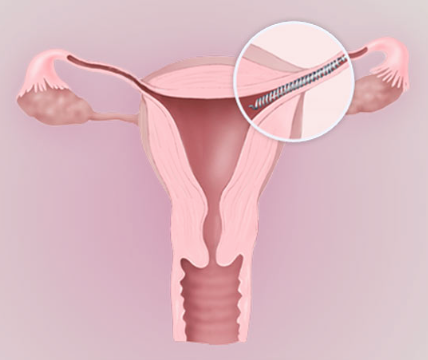
Undergoing a permanent birth control procedure can be a difficult decision for many women, and it is the manufacturer’s duty to adequately inform consumers about the risks – especially when these risks include chronic pain, tissue perforation, even death. In the case of Essure, a permanent, non-surgical birth control device inserted into the fallopian tubes, these risks, as well as many others, were not communicated. Essure was presented to women as an alternative to the more established procedure for permanent sterilization, tubal ligation, which requires surgery. However due to the rate of complications, Essure patients are 10 times more likely to require surgery in the year after receiving the implant than patients who opted for tubal ligation.
What is the Essure Medical Device and How Does it Work?
Essure is a non-surgical, permanent birth control device that was developed by Conceptus, Inc. (now a subsidiary of Bayer), and approved for the U.S. market in 2002. Metal coils are inserted into the fallopian tubes through the vagina and cervix without the need for general anesthesia or incisions. Over the next three months, scar tissue forms around the coils, creating a barrier that prevents sperm from reaching the eggs, thereby protecting the woman against unwanted pregnancy.
Essure is marketed as an effective, affordable, and convenient alternative to tubal ligation with a short recovery time. And unlike other popular contraceptive methods, Essure is hormone-free, making it an attractive option to those who cannot or do not wish to take hormones.
What are the Risks, Side Effects, and Problems?
Unfortunately, Essure also carries the risk of significant complications. Although the FDA has not recalled Ensure from the market, they issued a black box warning for the product, the strongest warning they can levy, due to the thousands of women who have already suffered from a range of chronic and debilitating side effects, including:
- Pelvic pain
- Migration of the device into the lower abdomen and pelvis
- Heavy menses or menstruation
- Fatigue
- Chronic headaches
- Weight gain and/or bloating
- Hair loss
- Perforation of the uterus or fallopian tubes
- Accidental pregnancies (including ectopic pregnancies)
- Death (including fetal death)
When and Why Should I Take Action?
If you or someone you know has been injured by the Essure implant, we recommend that you act as quickly as possible so that you can file your claim within the timeframe of Arizona’s statutes of limitations. McNamara Law Firm, PLLC has substantial experience representing defective medical product claims, including those affecting women, such as the Transvaginal Mesh device and the hormonal contraceptives Yasmin, Yaz and Ocella. Call us at 520-624-0126.
FDA Updates and Scientific Studies on Essure
- FDA takes additional action to better understand safety of Essure, inform patients of potential risks. Read more at FDA Safety Announcements.
- Essure Permanent Birth Control. Read more at FDA Products and Procedures.
- Safety and efficacy of hysteroscopic sterilization compared with laparoscopic sterilization: an observational cohort study. Read more at the British Medical Journal.
